The city’s junior high school graduates in 2024
The information of entrance examination for physical education has been released.
Let’s take a look at what changes have taken place.
one
Exam object
All the fresh and previous junior high school graduates who apply for ordinary high schools, secondary vocational schools and five-year higher vocational colleges in our city must take the physical education examination for junior high school graduates in 2024.
two
Examination time
From April to May, 2024, the specific time will be arranged by each county and city.
three
Examination items
Male and female students will take three exams each, and there will be mandatory and optional exams. In 2024, a one-minute skipping exam will be added.
1. Required items: 1000 meters for boys and 800 meters for girls.
2. Selection of test items: Candidates can choose two items from eight items (only one item can be selected for the "three big balls").
Boys: 50-meter running, standing long jump, throwing solid ball, pull-ups, skipping rope, basketball dribbling, football dribbling and volleyball padding.
Girls: 50-meter running, standing long jump, throwing solid balls, one-minute sit-ups, skipping rope, basketball dribbling, football dribbling and volleyball padding.
four
Test score
In 2024, junior high school graduates entered the physical education examination, with a full score of 60 points, which was included in the total score of the senior high school entrance examination. Among them: 25 points for a compulsory item; Choose two items, each with 17.5 points, totaling 35 points.
The starting point of each individual performance test is 0.
five
Detailed rules
Yichun city junior high school graduates in 2024
Rules of entrance examination for physical education
1. 1000-meter running and 800-meter running
(1) Site setting and equipment specifications
The venue is set in a closed 400-meter track and field with six or more circular runways. The marking lines required for the examination are clear, and obvious markers must be set at the starting line and the finishing line.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. The guide will take the candidates to the starting point in groups, and the number of candidates in each group will not exceed 20. Candidates will wear number clothes with timing chips as required, and the testers will check the candidates’ numbers one by one and enter the examination procedure after confirming that they are accurate.
Adopt a standing starting line and an arc starting line, regardless of the runway after starting; Follow the track and field rules of overtaking on the right. During the examination, the examinee has no foul phenomenon, and the record is valid.
Each candidate has only one chance to take the exam. Candidates can only take a make-up test on the same day with the consent of the examiner because of the foul of others or accidental fall during the test. Those who still violate the rules, the score will be 0. Candidates should complete the race within 7 minutes, and the time is recorded in minutes and seconds. Less than 1 second is not included in the score. If the student fails to finish the race within 7 minutes, the guide will take the candidate off the track, and the student’s score will be marked as "overtime", with a score of 0. When the exam is fouled, the result will be invalid.
(3) Foul behavior
1. Before starting, any part of the body touches or crosses the starting line;
2. Step into the inner edge of the runway during running;
3. Run in, push, pull and stop others from running in;
4. Run in by others, or run in with the help of others;
5. During the examination, take out the timing chip of the clothes.
(4) Matters needing attention
Candidates wear sneakers with rubber soles during the exam, and they are not allowed to wear spikes or leather shoes.
Second, 50-meter run
(1) Site setting and equipment specifications
There should be enough buffer space for several runways, and the ground should be flat soil or plastic ground. The marking lines required for the examination should be clear and marked on the corresponding passes. The starting line and the finishing line should be clearly marked. The system should meet the requirements of track and field competition rules.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates stand behind the starting line to prepare and listen to the sound of the equipment to start. Adopt a standing start. The instrument gives a prompt sound (each in position … about 2-3 seconds … gunshot prompt sound), and the candidates start. After reaching the finish line, confirm the results in the order of passes.
Candidates have only one chance to take the exam. Candidates can only take a make-up test on the same day with the consent of the examiner because of the foul of others or accidental fall during the test. Those who still violate the rules, the score will be 0.
(3) Foul behavior
Rush to run and cross the road during the exam.
(4) Matters needing attention
Candidates wear sneakers with rubber soles during the exam, and they are not allowed to wear spikes or leather shoes.
Third, the standing long jump
(1) Site setting and equipment specifications
Examination venues and equipment should be arranged on a flat ground. Use a special standing long jump tester.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates stand on the jumper and prepare for the exam. Both feet jump at the same time. The landing point must be in the test area, and the closest point of the body touching the ground from the jumper is the test score. No run-up or step jump is allowed.
Test twice in a row, and the electronic instrument automatically selects and stores the best performance record.
Candidates violate the rules in the exam, and the results are invalid; Those who have fouled twice and failed in the exam can only increase their chances once; Those who still violate the rules, the score will be 0.
(3) Foul behavior
1. Step on the line, run-up, step-by-step or jump.
2. After the test is completed, retreat or go out of the sensing area from the side, which is judged as a foul and the jumping result is invalid.
(4) Matters needing attention
After a single take-off and landing, you must go forward out of the test area and not backward, otherwise the test results may be affected.
Fourth, throw a solid ball
(1) Site setting and equipment specifications
A flat land 20 meters long and 5 meters wide; All counties and cities use solid balls of the same brand and specification, and the diameter of the ball is 14~14.5 cm; Weight: 2 kg (20 g) for both boys and girls; The sphere is cast by raw rubber; There must be no rolling animals in the ball. Use special laser rangefinder.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates stand behind the starting line to prepare for the exam. Before throwing, put your feet back and forth or left and right, and your body is facing the throwing direction. Raise the ball with both hands above your head and then lean back, with your shoulders parallel to the starting line, and throw the ball forward with both hands in situ; When the ball is released, the shoulders must be parallel to the starting line; Before the ball hits the ground, feet are not allowed to step on the line or throw it across the line, and all parts of the body are not allowed to touch the line.
Test twice in a row, and the rangefinder automatically selects and stores the best performance record.
Candidates violate the rules in the exam, and the results are invalid; Those who have fouled twice and failed in the exam can only increase their chances once; Those who still violate the rules, the score will be 0.
(3) Foul behavior
1. Failing to finish throwing from the original place, resulting in run-up, etc.
2. When testing, the foot steps on the line or any part of the body touches the ground in the landing area before the throwing line;
3. Throwing the upper limbs of the body to the throwing area or with one hand.
(4) Matters needing attention
1. Candidates wear sneakers with rubber soles during the exam, and they are not allowed to wear spikes or leather shoes.
2. At the moment when the solid ball is released, the foot can be pushed to the ground and off the ground at the same time (only at the moment of release). If the subject throws the ball with both feet in front and back, the back foot can take a step forward at the same time, but not step on the line.
Five, pull-ups (male)
(1) Site setting and equipment specifications
On the high horizontal bar, the horizontal bar used in the exam must meet the relevant requirements; According to the actual situation, venues with different heights can be set up for candidates to choose from; The laying of ground materials under the horizontal bar must fully consider the safety of candidates’ landing, and soft sand and gymnastics mats can be used.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates line up in a column in the specified area according to the test order. After hearing the number, the examinee jumps under the bar, holding the bar with both hands, hanging with both hands about shoulder width into a straight arm, and starting to do the first action after the body is in a static state; Bend the arm and pull the body up until the chin exceeds the upper edge of the bar, and complete it once after restoring the straight arm suspension; If the action does not reach this specification, it will not be counted; The examination time begins with holding the bar with both hands and ends with both hands off the bar. During this period, if the time interval between the two actions exceeds 10 seconds, the examination will automatically end. Candidates’ scores are counted according to the number of successful completion. Each candidate has only one chance to take the exam.
During the examination, the candidate commits a foul, regardless of the number of fouls, but can continue the examination.
(3) Foul behavior
1. Hands are not holding the horizontal bar;
2. Not starting from the static action;
3. Between the two movements, the arm is not fully straightened;
4. When the action is completed, the chin does not exceed the bar surface.
(4) Matters needing attention
When pull-ups, the body must not swing greatly, nor can it be propped up with other additional actions.
Six, 1 minute sit-ups (female)
(1) Site setting and equipment specifications
The venue is set on a flat ground, and candidates complete the test on instruments and equipment.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates lie on their back on the test instrument, with their hands and fingers crossed behind their heads, and at the same time, their arms are open, and the back of their hands and arms are touching the mat; Put your feet firmly, bend your knees, the big legs are at right angles, and your legs can be slightly separated; When sitting up, your elbows must touch or exceed your knees; When lying on your back, your shoulder blades must touch the pad. No external assistance is allowed during the test. Count the number of actions that the candidate completes in one minute that meet the standard. Each candidate only takes the test once.
Candidates have committed a foul, and the sit-ups done during the foul are not counted, but candidates can continue to take the exam.
When the exam is fouled, the result will be invalid.
(3) Foul behavior
1. When sitting up, the elbows do not touch or exceed the knees;
2. When lying on your back, the scapula did not touch the pad;
3. During the examination, the buttocks are off the mat.
(4) Matters needing attention
1. If it is found that the subjects sit up with the strength of elbow pads or hip ups and downs, it will not be counted this time.
2. Subjects’ feet must be placed on the mat.
Jump rope for seven or one minute.
(1) Site setting and equipment specifications
The examination venue is set on a flat ground, and intelligent instruments are used for testing. Candidates are tested in the arranged venue in groups. The skipping rope for the examination is special, and the handle contains timing and counting chips. Candidates are not allowed to use their own skipping rope, and there are several candidates in each group.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Candidates need to use the special skipping rope at the examination site, and the guide will take the candidates to the skipping field in groups. Candidates will stand in the designated area with signs as required and take the corresponding skipping equipment. Candidates will adjust the length of skipping rope to an appropriate length. After tying the shoelaces and preparing, the tester will check the candidates’ numbers one by one and enter the examination procedure after confirming that they are accurate.
Candidates listen to the instrument’s prompt tone of "Referee Ready, Athlete Ready, 5, 4, 3, 2, 1, Jump" to take the test. Candidates jump alternately with their feet or one foot in the test area, and each jump is valid once, and the test instrument automatically counts the time.
When the exam is finished, stand in the same place. Candidates can check the exam results at the right handle. After the equipment operator prompts them to shut down, gently put the rope on the exam position and exit in an orderly manner.
Candidates have only one chance to take the exam. If the examinee’s shoelaces fall off or are influenced by others during the examination, and the force majeure factors such as rope winding are confirmed by the invigilator, he can apply for a make-up jump opportunity.
(3) Foul behavior
1. Hands are not used to shake;
2. Jump and shake more;
3. Jumping out of the prescribed area and affecting others;
(4) Matters needing attention
1. Skipping rope and tripping during the test, except this time without counting, should be continued.
2. After the exam, candidates will lightly put the skipping rope to the designated position.
3. The skipping scene for the exam has been provided, and candidates are not allowed to bring their own skipping rope into the examination room.
Eight, basketball dribbling
(1) Site setting and equipment specifications
The court is set in the basketball court or on a flat plastic court. The setting requirements are as follows:
It is 20 meters long and 7 meters wide. 5 meters before the starting line, sign posts are set up, with 5 rows, 2 in each row; The distance between each row of sign poles is 3m, the distance between the center points of two sign pole bases in the same row is 1m, and the distance from the same side edge line is 3m. The starting area is 1 meter in the middle of the starting line. The setting method is as shown in the figure:
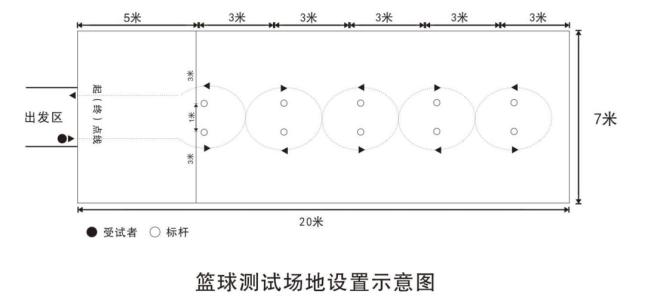
The height of the marker post is not less than 1.2 meters, the size of the test ball is No.6 basketball (its weight is 510~567g and its circumference is 724~737mm), and the air pressure in the ball meets the requirements of basketball competition rules.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. After hearing the number, candidates stand at the starting position of the starting (finishing) line with the ball and make preparations for departure. After giving the order, the examinee can move his feet and dribble the ball in the direction shown by the arrow in the figure. In the process of dribbling, if the candidate temporarily loses control of the ball, but the ball does not leave the test site, the candidate can pick it up by himself and return to the place where the ball is out of control to continue dribbling, and the timing will not stop. Candidates and the ball both return to the starting (final) line, and the timing stops. Each person tested twice and recorded good results.
Test scores are recorded in seconds, accurate to 1 decimal place (0. 1 second), and enter 1 when the second decimal place is not "0".
Candidates violate the rules in the exam, and the results are invalid; Those who have fouled twice and failed in the exam can only increase their chances once; Those who still violate the rules, the score will be 0.
(3) Foul behavior
1. Not starting from the designated area;
2. run away when you leave;
3. Both hands touch the ball at the same time during dribbling;
4. The dribbling height exceeds the examinee’s shoulder;
5. Body parts below the knee touch the ball;
6. Violations that are not allowed by other rules such as "wrist flip";
7. Leakage around the sign post;
8. Knock down the sign post;
9. During the examination, people or balls leave the test area;
10. When passing the finish line, people and balls are separated (candidates must touch the ball with one hand or both hands at the moment when they reach the finish line).
Nine, football dribbling
(1) Site setting and equipment specifications
The field is set on the flat ground in the middle of the track and field or on an independent football field. The surface is natural or artificial lawn (not smooth hard ground), and the fields are independent from each other to avoid mutual interference during the examination. The setting requirements are as follows:
It is 30 meters long and 10 meters wide, and obvious marking lines are set around the site. Sign poles are set up 5 meters before the starting line. The distance between the sign poles and the side lines on both sides is 5 meters, with a total of 5 poles, and the distance between the poles is 5 meters. One meter in the middle of the starting line is the departure area for candidates. The setting method is as shown in the figure:
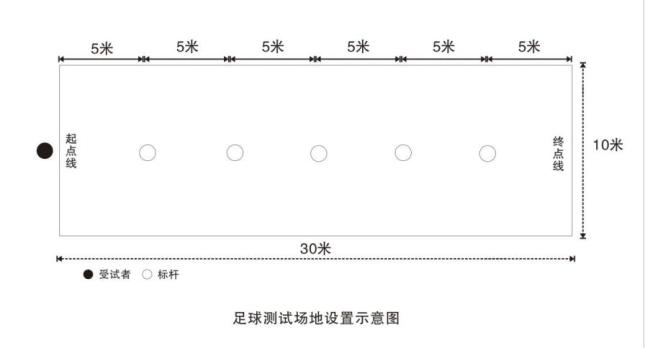
The height of the marker post is not less than 1.2m, the size of the test ball is No.5 football (its weight is 396~453g, and its circumference is 680~710mm), and the air pressure in the ball meets the requirements of football competition rules.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. After hearing the number, candidates will transport the ball to the starting area behind the starting line and make preparations for departure. After giving the order, the examinee dribbles forward and winds the pole in turn; After the ball passes the last post, the examinee touches the ball at least once and dribbles through the finish line; When both the man and the ball have crossed the finish line, the timing stops. Each person tested twice and recorded good results.
Record the test results in seconds, accurate to 1 decimal place (0. 1 second), and enter L when the second decimal place is not "0".
When the exam is fouled, the score will be invalid; Those who have fouled twice without results can only have one more chance; Those who still violate the rules will get 0 points for this score.
(3) Foul behavior
1. Not starting from the designated area;
2. run away when you leave;
3. handball;
4. Leakage around the sign post;
5. Knock down the sign post;
6. After the ball passed the last post, before reaching the finish line, the foot did not touch the ball;
7. Failing to complete the whole route as required;
8. During the test, the person or ball leaves the test area.
X. Volleyball cushion
(1) Site setting and equipment specifications
The venue is set on a flat and solid ground, and qualified test sites are encouraged to set the venue in the gymnasium. The setting requirements are as follows:
The length and width are 3 meters, and obvious marking lines are set around the site; A marker for judging the height of the cushion ball is set on the periphery of the site, and the vertical projection of the marker on the ground is 0.5 meters away from the sign line on the same side; The height of the marker should be set by considering the factors such as the counting method of instruments and equipment, the diameter of volleyball and so on to ensure that the height meets the requirements; Other matters needing attention in marker setting are determined according to the actual conditions of the selected instruments and equipment. The test sites for boys and girls should be set up separately.
The test ball is volleyball (soft volleyball is not allowed), its weight is 230~270g, its circumference is 650~670mm, and the air pressure in the ball meets the requirements of volleyball competition rules.
(2) Test methods
The guide will take the candidates to the examination venue in groups for identity confirmation (photo of the handheld education examination card is compared with my appearance) and check-in. Line up in a single column in the specified area according to the test order.
After hearing the number, the examinee enters the test area and throws the ball in place. After the first ball cushion is successful, the timer starts, and the individual continuously cushions the ball with both hands, requiring the correct hand shape, accurate hitting position and the specified height.
Each test should last no more than 1 minute. Each person takes the test twice, and records the better one.
If the cushion ball does not exceed the time limit, the ball lands or commits a foul, and the test is over; In case of overtime, the test will automatically end after the specified time. The height of the boys’ cushion ball (from the ground to the bottom of the ball) is not less than 2.24 meters, and the girls are not less than 2.00 meters. Only when the method is correct and the height meets the requirements can the cushion ball be counted. Count the number of cushion balls.
When the candidates take the exam, the ball touches the marker, and the consequences are borne by the candidates; In case of foul behavior, when the test is over, the number of cushion balls shall be based on the number recorded by instruments and equipment at the time of foul.
(3) Foul behavior
1. Touch the ball by passing the ball;
2. Step on the marking line or leave the test area.
(Slide down to see more)
six
grading standards
1. Strictly implement the grading standards for junior high school students’ physical health standards (revised in 2014).
2. Throwing a solid ball and skipping rope for one minute shall implement the grading standards of related items in junior middle school grade three in the National Physical Health Standard for Students promulgated and implemented by the Ministry of Education and the State Sports General Administration in 2007.
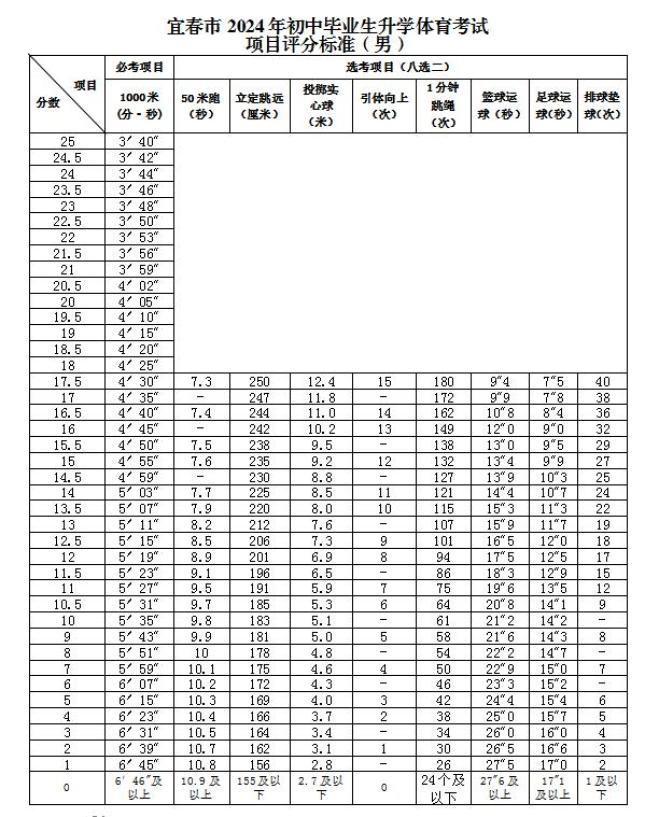
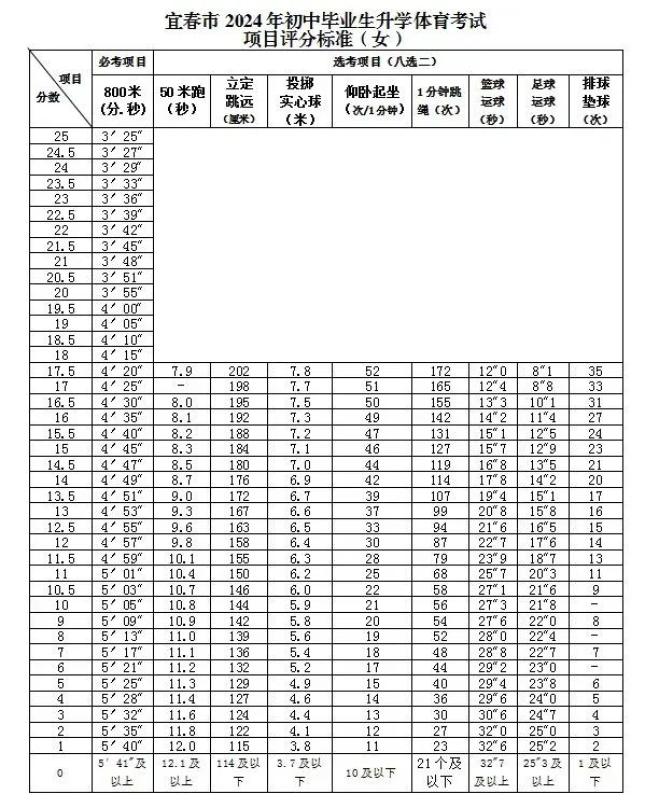
Source: Yichun Publishing
Editor in charge: Yuan Yonghua
Editor: Ao Juan
Issued by: Liu Ximing
Dear citizens:
Gao ‘an is creating a provincial civilized city! Please regulate parking, courteous to pedestrians, standardized operation, and no smoking in public places … Gaoan is more civilized because of you!

Help me order one
and
Share it with more people ↓↓↓↓ Continue to slide to see the next one.
Latest release! It is related to the high-quality Gao’ an in the 2024 senior high school entrance examination.

High-quality and high-praise sharing, reading and writing messages, sliding up to see the next one.
Original title: "The latest release! It is related to the 2024 senior high school entrance examination.
Read the original text



















