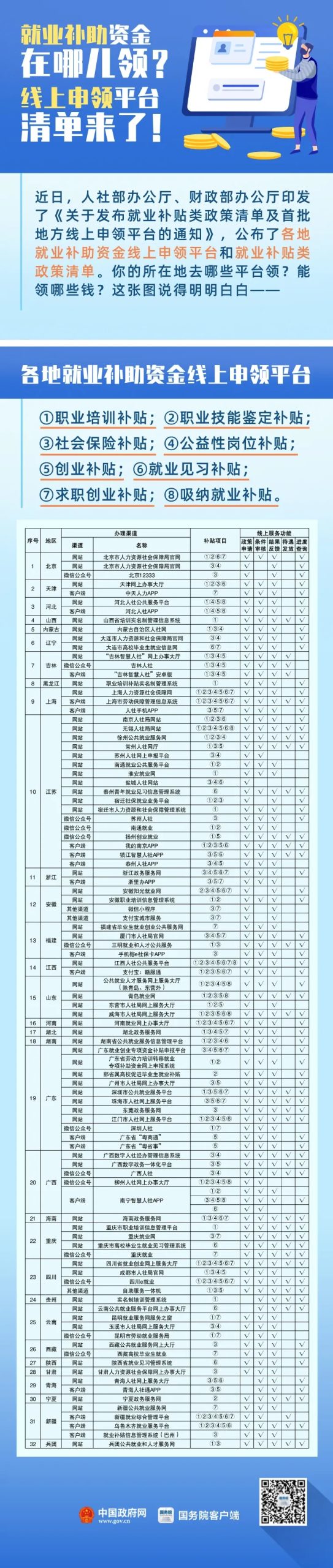
Who can get the subsidy?
What is the subsidy standard?
What is the specific application process?
↓↓↓
Attention enterprises! There are subsidies for absorbing employment!
(1) Vocational training subsidies
1. Subsidy target:
For enterprises that have newly hired children from poor families, college graduates in the graduation year, fresh junior high school graduates who have not continued their studies in urban and rural areas, rural migrant workers, and registered unemployed people in cities and towns, who have signed labor contracts for more than one year and participated in job skills training within one year from the date of signing the labor contract, they will be given vocational training subsidies to individual employees or enterprises after obtaining vocational qualification certificates.
Employees of enterprises who participate in new apprenticeship training and technician training and obtain vocational qualification certificates shall be given subsidies for individual employees or enterprises’ vocational training.
Enterprises, farmers’ professional cooperatives, poverty alleviation workshops and other production and business entities that absorb the employment of poor laborers and carry out training on behalf of workers will be given vocational training subsidies for no more than 6 months according to the number of people absorbed.
Enterprises affected by the COVID-19 epidemic, in the downtime, recovery period, organize employees to participate in online and offline training, according to the provisions included in the scope of subsidized training.
2. Subsidy standard:
Determined by the provincial people’s society and the financial department.
3. Application process:
Enterprises applying for job skills training, technician training and new apprenticeship training subsidies should provide tax invoices issued by training institutions to the human society department. Enterprises should report the training plan, the roster of trainers and a copy of the labor contract to the local community department for the record before carrying out technician training or new apprenticeship training.
After the audit of the human and social departments, the training subsidies or cost of living allowance funds applied by individuals shall be paid to the applicant’s own social security card bank account (or other bank account, which is chosen by the applicant independently, the same below) or personal credit account according to regulations; For enterprises and training institutions to apply for or directly supplement the training subsidy funds of training institutions, they shall be paid to the bank accounts of enterprises and training institutions.
(2) Social insurance subsidies
1. Subsidy target:
Units that employ people with employment difficulties and pay social insurance premiums. (Persons with employment difficulties refer to those who find it difficult to achieve employment due to physical condition, skill level, family factors, land loss and other reasons, and those who have been unemployed for a certain period of time and have not yet achieved employment. The specific scope is determined by the provincial people’s governments and dynamic adjustment is implemented.)
Units that place people with employment difficulties through public welfare posts and pay social insurance premiums. And units that resettle personnel through temporary public welfare posts such as disinfection and epidemic prevention, cleaning and sanitation (the deadline for acceptance is December 31, 2020).
Recruit college graduates who have not been employed within 2 years after leaving school, sign labor contracts for more than 1 year and pay social insurance premiums.
2. Subsidy standard:
Units that recruit or place people with employment difficulties shall be subsidized according to the basic old-age insurance premium, basic medical insurance premium and unemployment insurance premium actually paid by them, excluding the individual contribution. The subsidy period can be extended to retirement except for those who are less than 5 years away from the statutory retirement age, and the rest of the staff shall not exceed 3 years at the longest. During the period of phased reduction and exemption of social insurance premiums, the social insurance subsidies for enterprises to absorb people with employment difficulties may be postponed. The term of social security subsidy for temporary public welfare posts shall not exceed 6 months.
After the expiration of the public welfare post, it is still difficult to achieve employment through other channels. Older people with employment difficulties, zero-employment family members, severely disabled people and other special difficulties can be resettled through public welfare posts again according to procedures. The subsidy period is recalculated and submitted to the provincial people’s society and the financial department for the record. In principle, the cumulative number of resettlement times is not more than 2 times. From January 1 to December 31, 2020, if the policy of engaging in public welfare posts has not yet achieved stable employment, the policy enjoyment period can be extended by one year.
For small and micro enterprises that recruit qualified college graduates, subsidies will be given according to the basic pension, basic medical care and unemployment insurance premiums actually paid by college graduates, excluding the individual contributions, and the maximum period will not exceed one year.
3. Application process:
Social insurance subsidies shall be paid first, and the following requirements shall be followed according to the specific use of funds:
Units that employ people with employment difficulties and small and micro enterprises that employ qualified college graduates shall provide the following materials to local social departments: basic identity certificates (including ID cards, employment and entrepreneurship certificates, employment and unemployment registration certificates, social security cards, whichever is available, the same below) or copies of graduation certificates and labor contracts.
Units that place people with employment difficulties through public welfare posts shall provide copies of basic identity certificates to local community departments when applying for social insurance subsidies.
After the audit by the human and social departments, the subsidy funds will be paid to the bank account of the unit.
(3) Public welfare post subsidies
1. Subsidy target:
People with employment difficulties who are placed in public welfare posts, poor laborers who set up files and set up cards, and people who are placed in temporary public welfare posts such as disinfection and epidemic prevention, cleaning and sanitation developed in various places (the deadline for acceptance is December 31, 2020).
2. Subsidy standard:
The public welfare post subsidy standard shall be implemented with reference to the local minimum wage standard. The maximum subsidy period is not more than 3 years, and it can be extended to retirement if it is less than 5 years away from the statutory retirement age. After the expiration of the subsidy, it is still difficult to achieve employment through other channels, such as elderly people with employment difficulties, zero-employment family members, severely disabled people and other people with special difficulties. They can be resettled again through public welfare posts according to procedures, and the subsidy period will be recalculated and submitted to the provincial people’s society and the financial department for the record. In principle, the cumulative number of resettlement times will not exceed 2 times. From January 1 to December 31, 2020, if the policy of engaging in public welfare posts has not yet achieved stable employment, the policy enjoyment period can be extended by one year.
The rural public welfare post subsidy standard is determined according to factors such as labor time and labor intensity, and in principle it is not higher than the local urban public welfare post subsidy level. For the resettlement personnel, the longest term of the labor contract or labor service agreement signed is not more than one year.
The subsidy standard for temporary public welfare posts is determined according to the work tasks and working hours, and the maximum subsidy period is no more than 6 months.
3. Application process:
Units that resettle people with employment difficulties through public welfare posts and set up files for poor laborers should provide the following materials to the local social departments when applying for public welfare post subsidies: a copy of the basic identity certificate, a detailed account of wages paid by the unit, etc.
After the audit by the human and social departments, the subsidy funds will be paid to the bank account of the unit or the bank account of the social security card of the public welfare post placement personnel.
(4) Employment trainee subsidy
1. Subsidy target:
Units that recruit unemployed college graduates and unemployed youth aged 16-24 who have left school for 2 years to participate in employment internships.
2. Subsidy standard:
Determined by the provincial people’s society and the financial department. Subsidies are used by trainee units to pay the basic living expenses of trainees during their probation, to handle personal accident insurance for trainees, and to guide and manage the trainees.
The trainee probation period retention rate reached more than 50% of the units, can be appropriately raised trainee subsidy standards.
If the probation is temporarily suspended due to the epidemic situation, the subsidy period of the probation unit shall be extended accordingly. For those who sign labor contracts with college graduates before the probation period, the probation unit shall be given a probation subsidy for the remaining period.
3. Application process:
The trainee unit provides the following materials to the local community department: a copy of the basic identity certificate (or graduation certificate), an employment internship agreement, a detailed account (form) for the basic living allowance issued by the unit, and a copy of the personal accident insurance invoice for the trainee.
After the audit by the human and social departments, the subsidy funds will be paid to the bank account of the unit.
(five) a one-time absorption of employment subsidies
1. Subsidy target:
Enterprises that recruit registered unemployed people for more than half a year and sign labor contracts for more than one year and pay social insurance according to regulations (the implementation period is 2020);
Enterprises that started production and distribution of urgently needed materials for epidemic prevention and control during the Spring Festival in 2020 (as of February 9);
Small and medium-sized enterprises that employ college graduates in the graduation year and sign labor contracts for more than one year (the deadline for policy acceptance is December 31, 2020).
2. Subsidy standard:
Determined by the provincial people’s society and the financial department.
3. Application process:
Determined by the provincial people’s society and the financial department.
Employment and entrepreneurship also have subsidies!
(1) Vocational training subsidies
1. Subsidy target:
Children from poor families, college graduates in graduation year, fresh junior high school graduates who have not continued their studies in urban and rural areas, rural migrant workers, and registered unemployed people in cities and towns have participated in employment skills training and entrepreneurship training, and obtained vocational qualification certificates after training (or vocational skill grade certificates, special vocational ability certificates, and training qualification certificates).
2. Subsidy standard:
It is determined by the provincial people’s society and the financial department according to the training cost, training duration, market demand and obtaining relevant certificates.
Rural students and urban low-income family students who participate in the labor preparation training, as well as those with employment difficulties and zero-employment family members who participate in the training in 2019 and 2020, will be given certain cost of living allowance during the training period.
3. Application process:
The above-mentioned personnel provide the following materials to the local community department: the original or photocopy of the basic identity certificate, the tax invoice issued by the training institution (or the administrative fee bill, the same below), etc.
After the audit of the human and social departments, the training subsidies or cost of living allowance funds applied by individuals shall be paid to the applicant’s own social security card bank account (or other bank accounts, which shall be chosen by the applicant independently, the same below) or personal credit account according to regulations.
(two) occupation skill appraisal subsidies
1. Subsidy target:
Children of poor families who have passed the initial vocational skill appraisal and obtained the vocational qualification certificate (excluding the training certificate), college graduates in graduation year, junior high school graduates who have not continued their studies in urban and rural areas, migrant workers in rural areas, and registered unemployed people in cities and towns.
2. Subsidy standard:
Determined by the provincial people’s society and the financial department. For those who are included in the guidance catalogue for the evaluation of vocational qualifications and vocational skills in key industries, the subsidy standard may be appropriately raised.
3. Application process:
The above-mentioned personnel provide the following materials to the local community department: the original or photocopy of the basic identity certificate, the tax invoice (or administrative fee bill) issued by the vocational skill appraisal institution, etc.
After the human and social departments review, the subsidy funds will be paid to the applicant’s own social security card bank account.
(3) Social insurance subsidies
1. Subsidy target:
Flexible employment of people with employment difficulties, flexible employment of college graduates who have not been employed within 2 years after leaving school.
2. Subsidy standard:
According to the social insurance premium paid after flexible employment, a certain amount of social insurance subsidy shall be given, which shall not exceed 2/3 of the actual payment in principle.
The term of social security subsidies for people with employment difficulties shall not exceed 3 years at the longest, and may be extended to retirement for those who are less than 5 years away from the statutory retirement age. The maximum period of social security subsidies for college graduates shall not exceed 2 years. From January 1 to December 31, 2020, if the subsidy policy expires and stable employment has not been achieved, the policy enjoyment period can be extended by one year.
3. Application process:
The above-mentioned personnel shall provide the original or photocopy of the basic identity certificate and the flexible employment certificate to the local community department.
After the human and social departments review, the subsidy funds will be paid to the bank account of the unit or the bank account of the applicant’s own social security card.
(4) One-time job-seeking and entrepreneurship subsidies
1. Subsidy target:
College graduates and secondary vocational school graduates from low-income families, poor disabled families, poverty-stricken families and extremely poor people who have the will to find jobs and start businesses in the graduation school year, disabled college graduates and secondary vocational school graduates who have obtained national student loans. Hubei universities and graduates from Hubei universities in 2020.
2. Subsidy standard:
Determined by the provincial people’s society and the financial department.
3. Application process:
Eligible graduates apply for job-seeking and entrepreneurship subsidies in their schools, and should provide local social departments with proof materials, copies of student status certificates, etc. for graduates to obtain national student loans (or poor families with subsistence allowances, physical disabilities, poverty-stricken disabled families, and poor relief and support). The application materials shall be subject to the preliminary examination and publicity of the school where the graduates are located.
After the audit by the local community department, the subsidy funds will be paid to the bank account of the graduate’s own social security card.
(5) One-time business start-up subsidy
1. Subsidy target:
In qualified areas, small and micro enterprises are established for the first time or engaged in self-employment, and the established enterprises or individual industrial and commercial households have been operating normally for more than one year since the date of industrial and commercial registration, college graduates, people with employment difficulties and entrepreneurs who have returned to their hometowns within two years. One-time business start-up subsidies for poor laborers will be relaxed to normal operation for more than 6 months.
2. Subsidy standard:
Determined by the provincial people’s society and the financial department.
3. Application process:
Determined by the provincial people’s society and the financial department.
More employment services ~ ~ ~ ~ ~ ~ ~
What employment support was provided during the epidemic? How do migrant workers return to work? Where can I apply for unemployment benefits? In order to make it convenient for everyone to know the latest employment policies and obtain convenient employment services in time, we jointly launched the epidemic response employment service area with the Ministry of Human Resources and Social Security, and the client applet was officially launched in the State Council.
Scan sunflower code to enter the employment service area















































 18-inch hub
18-inch hub 19-inch hub
19-inch hub