In autumn and winter, respiratory viruses once came to the fore: influenza, mycoplasma pneumonia, respiratory syncytial virus infection and other respiratory infectious diseases frequently occurred, high fever and cough came one after another, and the medical system was overwhelmed. Industry experts have repeatedly called for prevention to outweigh treatment, and for respiratory infectious diseases, safe and effective vaccines should be the first line of defense to protect the public. During the Spring Festival, the whole family is reunited and the guests are crowded. At the same time, we should also be alert to the respiratory virus to "join in the fun". The Infectious Diseases Management Office of China CDC also advises people to do a good job in self-health monitoring, maintain good hygiene habits, and timely vaccinate against influenza, pneumonia and other vaccines to protect themselves and their relatives and friends.
Clover Bio, focusing on building a leading respiratory vaccine product portfolio, launched the brand promotional film "Breathing Everything in Three Living Things" today. The whole film takes "guarding breath" as the main line, telling the story of clover creatures and sincerely telling the social responsibility of a local innovative enterprise. At the beginning of the new year, clover creatures offer their blessings here, and they are willing to continue to protect people’s respiratory health with innovative vaccine products!
A glance at the brand-new promotional film of Clover Bio;
From the beauty of "one breath and one breath" to the suffocation anxiety of "respiratory infectious diseases raging", "When the breath of life is threatened, who will protect the breath of everything?" Clover has been deeply involved in the field of respiratory vaccines, hoping to help the public build an immune barrier against respiratory diseases and protect the public with high-quality and safe vaccines.
Who is the clover creature?
Clover is committed to saving lives and improving global health with innovative vaccines, and bringing innovative vaccines to the world and benefiting more people by using revolutionary science and global partnership. With comprehensive R&D strength, production and commercialization capabilities, and strong partnership with relevant institutions distributed around the world, Clover Bio has developed a variety of candidate vaccine pipelines, in the hope that innovative vaccines can prevent more diseases and help reduce the burden of public health.
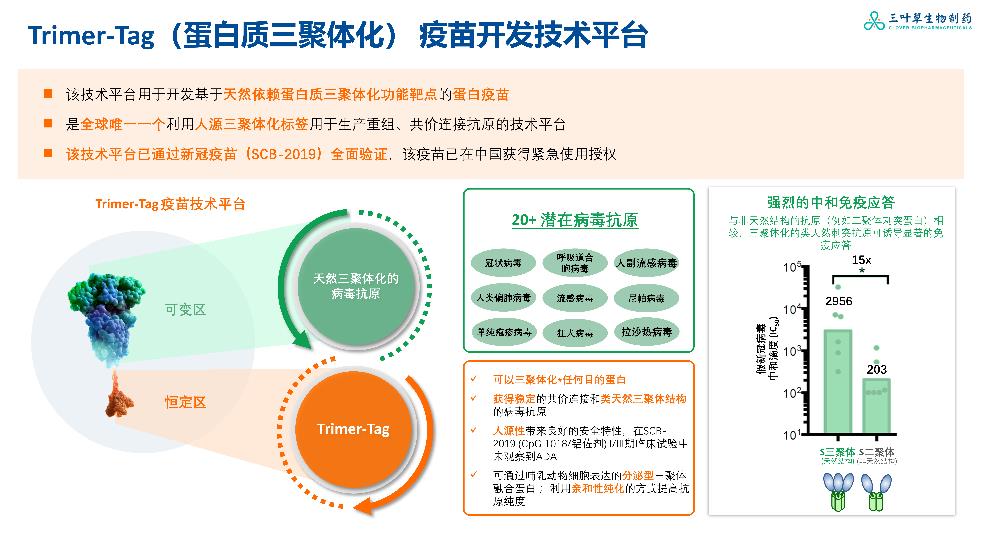
Source innovation
Clover insists on source innovation and has an original and leading Trimer-Tag vaccine research and development platform in protein, which is the only technology platform in the world that uses human trimer-tag to produce recombinant and covalently linked antigens. The technical platform of clover biology has been fully verified by its first commercial vaccine product, and its applicability can be extended to other product pipelines under research. In addition, Clover Bio has proven global vaccine research and development strength, and its research experience spans five continents (eight countries), and it has a global scientific advisory committee with a wide professional background, which injects vitality into the research and development of innovative self-developed pipelines.

Global cooperation
Clover creatures actively explore and cooperate with reputable partners around the world; In early 2023, it signed an exclusive agreement with Guoguang Biotechnology Co., Ltd. to distribute tetravalent influenza vaccine AdimFlu-S (QIS) in Chinese mainland. Clover has also received $397 million from the Epidemic Prevention Innovation Alliance (CEPI) to support the research and development of COVID-19 vaccine SCB-2019. At present, Clover Bio is still actively exploring new strategic relationships with the world’s top biopharmaceutical companies, biotechnology companies and academic institutions to help realize its pursuit of innovative vaccines.

Commercial production
Clover creatures are persistent and focused, and have established and developing commercial production capacity. Clover Bio’s own factory in Changxing, Zhejiang Province, which is ready for commercialization, has passed the GMP verification in China for many times and obtained the vaccine drug production license (DML) issued by Zhejiang Drug Administration, which is ready for the commercial production of self-developed pipeline products. At the same time, in addition to its own commercialization team, Clover Bio has also reached a strategic cooperation with Keyuan Trade, and will use its extensive sales and distribution network to supplement its own commercialization capabilities.
Product pipeline
Clover bio-toughness polishing, and strive to create a leading respiratory vaccine product portfolio. At present, there are two vaccine products in the commercialization stage (namely tetravalent seasonal influenza vaccine and COVID-19 vaccine), and the candidate vaccine SCB-1019 of respiratory syncytial virus (RSV) in its self-developed pipeline has a strong momentum. At present, clover has successfully developed a highly stable trimer antigen, which is close to the natural sequence and the natural pre-fusion F(Pre-F) structure, and has applied for a patent. According to its recently published preclinical trial data, SCB-1019 shows a higher neutralizing antibody and a higher quality antibody level than the other two RSV vaccines that have been commercialized internationally. And thanks to its fully humanized protein Trimer-Tag vaccine technology platform and many clinical trial experiences based on the past vaccine development, it is expected that there will be no pre-existing immunity, which has great potential to meet the annual and seasonal vaccination needs of RSV vaccine; At the same time, SCB-1019 of clover will not use oil-in-water emulsified adjuvant, or it can show excellent safety and tolerance characteristics. In the next step, clover will accelerate the clinical application and development, and look forward to the clinical progress of more RSV candidate vaccines in 2024.
"Clover biology is based on source innovation, focusing on respiratory vaccine products and believing in the power of accumulation. As a local innovative enterprise, Clover Bio shoulders social responsibility, protects the public with innovative vaccine products and helps China and global health. " When interviewed by Mr. Liang Guo, CEO and executive director of Clover Bio, everything he said shows that innovative entrepreneurs are willing to feed back the society and protect the public with innovative power. "The root of clover’s insistence on source innovation is based on the protein Trimer-Tag vaccine research and development platform I invented, which is a technology to secretively express the trimer-like antigen of natural virus with covalently stable disulfide bonds by using the human natural protein trimer tag," explained Dr. Liang Peng, founder, chairman and chief scientific officer of clover. "The surface antigens of many RNA viruses (such as Covid-19, respiratory syncytial virus, influenza virus, rabies virus, etc.) that invade human beings are presented in the form of natural trimer structure, and the essence of Trimer-Tag technology comes from the most abundant collagen trimer structure in human body, which can trimerize any fusion protein, thus obtaining stable virus surface antigens with natural trimer-like structure; In addition, human nature brings good safety characteristics, and Trimer-Tag technology has strong versatility in the development of recombination vaccines. " Dr. Liang Peng added, "In addition to our self-developed and commercialized COVID-19 vaccine,The prospect of RSV vaccine in clinical phase I, which is also independently developed based on Trimer-Tag technology platform, is also optimistic in the industry. Dr. Liang’s patient explanation cannot hide his persistence in scientific exploration and his cherish of hard-won research and development achievements. "In clover biology, we have never stopped exploring in research and development. The so-called source is long, the roots are deep, and the source innovation is our root."